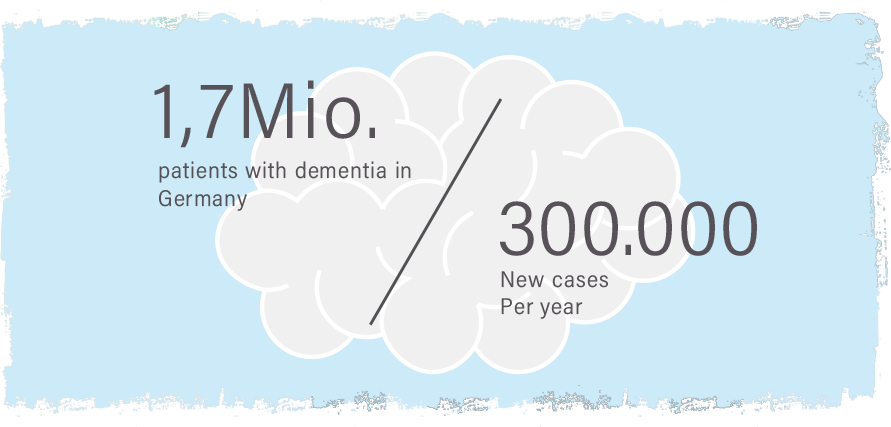
In Germany, around 1.7 million people have Alzheimer’s disease or another dementia disorder. This is a term used to describe various conditions in which mental performance declines quite sharply over time. By far the majority of those affected have Alzheimer’s. More than 300,000 new cases occur every year.
There is no drug or therapy that can cure Alzheimer’s disease. Medication can at best delay or somewhat alleviate the symptoms. So far, there is also no preventive vaccination. However, scientists are working on vaccines against Alzheimer’s disease. Fellbach-based biosyn Arzneimittel GmbH is playing a key role in this work. The biotechnology company supplies the carrier KLH for a potential Alzheimer’s vaccine that is currently under development.
Vaccination is a targeted stimulation of the immune system that elicits a specific immune response. In this process, antigens are administered to set the immune system in motion against the pathogen to be fought. Antigens are therefore substances that cause a defense reaction of the body. This results in the formation of antibodies, which are produced by immune cells against foreign antigens or antigens that are recognized as a threat. When antigen and antibody combine, a reaction occurs: the cell to be attacked is destroyed.
In the case of KLH, the advantage is that this carrier substance is per se an extremely strong antigen on whose surface weak antigens can be chemically coupled. The immune system then reacts both to the KLH and to the weak antigens, which gain significantly in antigenicity through KLH and thus become recognizable to the immune system. biosyn Arzneimttel GmbH manufactures the carrier under the recognized rules of Good Manufacturing Practice (GMP), which make it suitable for use in vaccines administered to humans.
The carrier protein KLH is used in various vaccines currently being tested, for lymphoma or Alzheimer’s disease. What are the chances now for an Alzheimer’s vaccine? Now that phase II of the clinical trial has been successfully completed and significant indications of an improvement in Alzheimer’s patients have been achieved, there is optimism at biosyn Arzneimittel GmbH: because there is a good chance that the phase III trial will also be successfully completed. The aim of such registration studies is to test the efficacy and tolerability of a new active substance in comparison with a placebo. Should this prove positive, there would be nothing standing in the way of worldwide approval of this vaccine.
Click here for more information: